Stop using Delsam Pharma’s Artificial Eye Ointment
Delsam Pharma’s Artificial Eye Ointment is sold over-the-counter. Anyone who has it should discontinue its use and seek medical attention for any eye infections.
The FDA had previously issued warnings about EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears over contamination concerns. Both are manufactured by Global Pharma Healthcare.

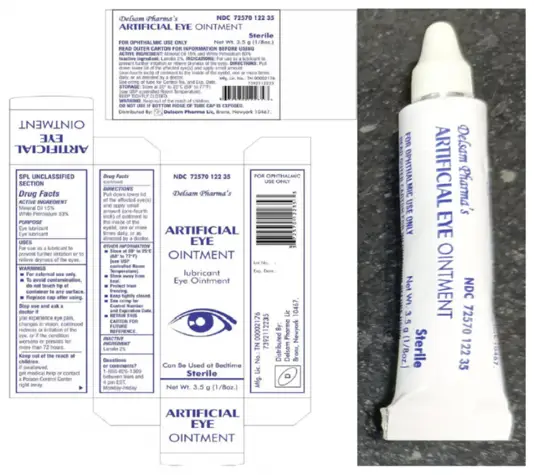
Delsam Pharma’s Artificial Eye Ointment
Using the product could cause an eye infection that could lead to permanent blindness, according to the FDA recall.
FOR IMMEDIATE RELEASE – February 24, 2023, Global Pharma Healthcare is voluntarily recalling Batch No. H29 of Artificial Eye Ointment, distributed by Delsam Pharma to the consumer level, due to possible microbial contamination. Additionally, some product packaging is leaking or may otherwise be compromised.
Risk Statement: Use of contaminated eye ointment may cause adverse events, including infection in the eye that could lead to blindness. To date, Global Pharma Healthcare has not received any reports of adverse events related to this product.
Artificial Eye Ointment (mineral oil 15%, white petrolatum 83%, 3.5 grams / 1/8 oz.) is used as an eye lubricant and to relieve dryness of the eyes. The affected product is packaged in a white aluminum tube within a paper carton. The product can be identified by the photos provided below. The product was distributed nationwide in the United States, and by Delsam through internet retail sites. Delsam Pharma’s NDC for this product is 72570-122-35, and its UPC code is 3 72570 12235 3.
Global Pharma Healthcare is notifying the brand owner and importer of this product, Delsam Pharma, about this recall, and is requesting that wholesalers, retailers, and customers who have the recalled product should stop any use and discard the product safely and appropriately.
Reference : FDA