Bausch Lomb TENEO™ Excimer Laser Platform for Myopia and Myopic Astigmatism LASIK Vision Correction Surgery Receives FDA Approval
JANUARY 8, 2024
First Excimer Platform Approved in Nearly Two Decades Offers Accuracy, Efficiency and Usability Advantages
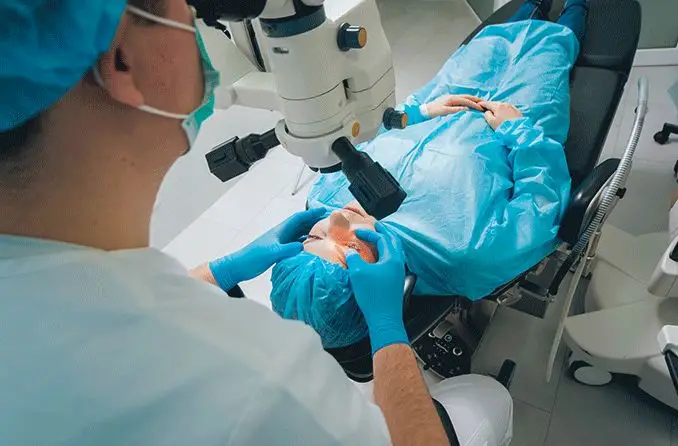
Bausch Lomb TENEO™ Excimer Laser
VAUGHAN, Ontario-Bausch + Lomb Corporation (NYSE/TSX: BLCO), a leading global eye health company dedicated to helping people see better to live better, today announced the U.S. Food and Drug Administration (FDA) has approved the TENEO Excimer Laser Platform for laser-assisted in situ keratomileusis (LASIK) vision correction surgery for myopia and myopic astigmatism (nearsightedness and nearsightedness with astigmatism)*.
“TENEO has been well received and is widely adopted in more than 50 countries around the world, and now U.S. ophthalmologists will benefit from this versatile laser,” said Luc Bonnefoy, president, Global Surgical, Bausch + Lomb. “The precise engineering of this platform delivers a fast, small, technologically advanced machine that provides an exceptional experience for both surgeons and patients.”
TENEO offers several unique features not offered by previous excimer laser platforms.
Accuracy
The advanced eye-tracker operates at 1,740Hz, which is more than three times the speed of the laser’s repetition. This feature helps to ensure the laser ablation pattern is not negatively impacted by a patient’s eye movement and helps achieve outstanding post-operative outcomes.
The platform’s high-speed laser operates at 500Hz, the fastest ablation time of all excimer lasers available in the United States at approximately 1.2 seconds per diopter.
Bausch Lomb TENEO™ Excimer Laser Efficiency
The platform’s customizable graphical user interface touchscreen simplifies set up and allows surgeons to access to the patient data they choose without flipping through screens.
The cutting edge TENEO software treats the manifest refraction and does not require a nomogram therefore streamlining surgical planning by eliminating several steps prior to treatment. The logical, intuitive process requires three steps: select patient; choose and confirm treatment; treat.
In addition to these time saving advantages, the compact design of the TENEO (0.6m2 (6.8 sq. ft)) makes it the smallest excimer laser unit available in the United States, freeing up limited clinical space, which is a critical concern for many surgeons.
LaZrPlastique: LASIK without cutting the cornea
Usability
TENEO has been specifically engineered for the comfort of both surgeons and patients. It features a 360o swivelling microscope that adapts to surgeon height and posture, aiding in surgeon comfort.
The treatment bed comfortably accommodates patients of all sizes, swings out for easier access, and can be customized for optimal head positioning. The bed can be swivelled to be positioned for a second treatment device† so that patients do not have to get up and move, enhancing comfort and simplifying the surgical process.
“FDA approval of TENEO represents a major milestone for the advancement of laser vision correction technology in the United States,” said George Waring IV, M.D., ophthalmologist and founder and medical director, Waring Vision Institute, Mt. Pleasant, S.C. “In addition to the technological advantages TENEO offers, the open air feeling around the laser and quiet performance contribute to a comfortable experience for the patient.”
For more information, visit https://www.bauschsurgical.com/refractive/teneo/.
Indications and Important Safety Information for Technolas Teneo 317 Model 2 System
Indications for Use. The Technolas Teneo 317 Model 2 is indicated for laser-assisted in situ keratomileusis (LASIK) in: (1) Patients for the reduction or elimination of myopic astigmatism up to -10.00 D MRSE, with sphere between -1.00 D and cylinder between 0.00 and -3.00 D; (2) Patients who are 22 years of age or older; (3) Patients must have a stable refraction in the last 12 months, as documented by previous clinical recordings, i.e., the spherical and cylindrical portions of the manifest distance refraction have not progressed at a rate of more than 0.50 D per year prior to the baseline examination in the eye(s) to be treated.
WARNING. Danger of injury due to failure to observe the patient selection criteria! Failure to observe the contraindications and potential adverse effects may result in serious permanent patient injury. The usage of the laser system is limited to a specific field of applications. Observe the contraindications and potential adverse effects listed in the User Manual before selecting a patient and starting any treatment.
Contraindications. Contraindications of the Technolas Teneo 317 Model 2 include patients:
- (1) with any type of active connective tissue disease or autoimmune disease;
- (2) with signs of keratoconus, abnormal corneal topography, and degenerations of the structure of the cornea (including but not limited to pellucid marginal degeneration);
- (3) with significant dry eyes (severe Dry Eye Syndrome). If patients have severely dry eyes, LASIK may increase the dryness. This may or may not go away. Severe eye dryness may delay healing of the flap or interfere with the surface of the eye after surgery. It may result in poor vision after LASIK;
- (4) for whom the combination of their baseline corneal thickness and the planned operative parameters for the LASIK procedure would result in less than 250µ of residual corneal thickness from corneal endothelium;
- (5) with uncontrolled diabetes;
- (6) with uncontrolled glaucoma;
- (7) with active eye infections or active inflammation:
- (8) with recent herpes eye infection or problems resulting from past infections;
- (9) with known sensitivity to medications used for standard LASIK surgery.
Potential Risks and Side Effects:
- (1) Miscreated flap;
- (2) Subconjunctival hemorrhage or bleeding;
- (3) Wrinkles in flap that may require a flap lift;
- (4) Corneal erosion/abrasion, epithelia defect;
- (5) Elevated IOP;
- (6) Debris or foreign body under flap;
- (7) Epithelial ingrowth under flap;
- (8) Debilitating visual symptoms, especially at night;
- (9) Decreased or fluctuating visual acuity;
- (10) Decreased ability to see in low-light conditions;
- (11) Light sensitivity;
- (12) Dry Eye syndrome;
- (13) Inadequate treatment result;
- (14) Regression;
- (15) Corneal damage;
- (16) Posterior vitreous detachment or retinal detachment, floaters or vascular accidents; (17) Foreign body sensation or pain (initial postoperative days); also, potentially including chronic eye pain that is resistant to therapy referred to as neuropathic pain;
- (18) Infection/inflammation;
- (19) CTK (Central Toxic Keratopathy);
- (20) Medication intolerance; (21) Ptosis; (22) Cataract; (23) Ocular penetration; (24) Potential risk of psychological harm.
Discover more from An Eye Care Blog
Subscribe to get the latest posts sent to your email.

You must be logged in to post a comment.