Study Suggests High Frequency Electrical Noise Results in Congenital Night Blindness
In a groundbreaking resolution to a three-decade-long enigma, neuroscientists at Johns Hopkins Medicine have utilized genetically modified mice to decipher how a specific mutation in the gene for the light-sensitive protein rhodopsin induces congenital stationary night blindness.
This condition, present from birth, severely impairs vision in low-light environments.
Published on May 14 in the Proceedings of the National Academy of Sciences, the research illuminates that the rhodopsin gene mutation, known as G90D, generates an atypical background electrical noise. This noise desensitizes the retina’s rods—cells crucial for night vision—resulting in night blindness.
The identification of this peculiar electrical activity could pave the way for future therapeutic targets, as highlighted by the study’s authors.
King-Wai Yau, Ph.D., a professor in the Department of Neuroscience at Johns Hopkins University School of Medicine, notes that these electrical phenomena could enhance scientific comprehension of the functioning of rods and cones in the eye.
The research was spearheaded by Yau and postdoctoral fellow Zuying Chai.
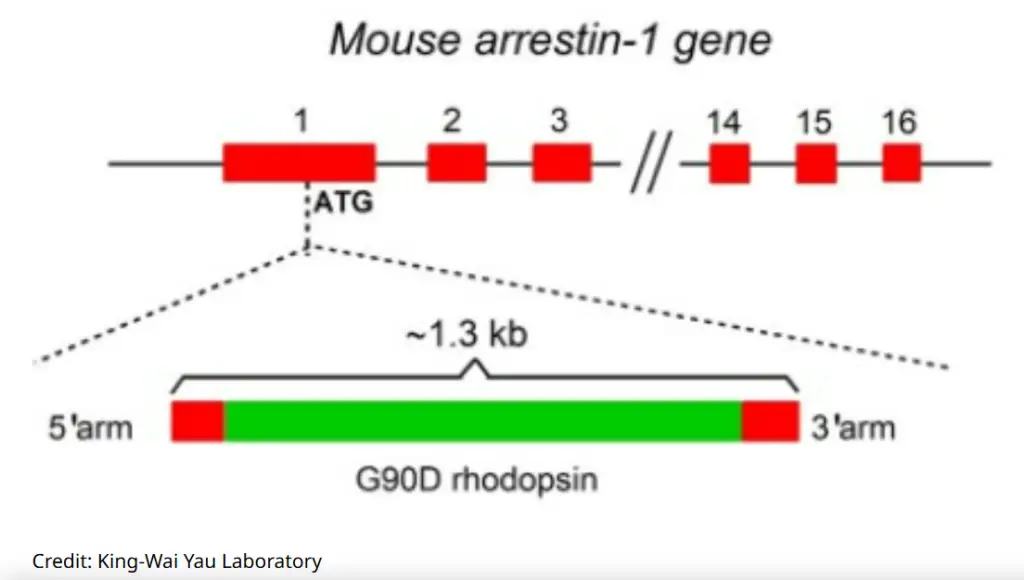
Despite awareness of the G90D mutation’s ability to produce background electrical noise that desensitizes rods, the nature and precise molecular origin of this ‘noise’ remained unresolved for nearly 30 years. Yau asserts that using a mouse model with a minimal expression level of G90D rhodopsin has helped elucidate the mechanism behind this disease.
Comparing the low expression level of G90D in genetically engineered mice with the level found in human patients, the researchers concluded that the low amplitude yet exceedingly high frequency electrical activity might be the primary contributor to night blindness in humans.
High Frequency Electrical Noise Results in Congenital Night Blindness-study
Apart from this unusual electrical noise, rhodopsin is also known for producing another type of electrical activity called spontaneous thermal isomerization. Here, the thermal energy within the rhodopsin molecule sporadically activates it. Unlike the low amplitude, high frequency noise, the spontaneous isomerization of G90D rhodopsin exhibited high amplitude but low frequency. The experiments revealed that the spontaneous isomerization rate of G90D rhodopsin is approximately two hundred times higher than that of normal rhodopsin, yet its effect on rod adaptation is insufficient to significantly contribute to night blindness in humans.
Under typical conditions, rods are highly sensitive to light. However, in individuals with night blindness, rods fail to detect light changes accurately, rendering them ineffective in darkness. Such individuals require brighter light to see in dim environments, Yau explained.
For decades, despite knowledge of the G90D mutation, researchers struggled to determine its exact mechanism in causing night blindness. Previous mouse models with this mutation produced high levels of background noise akin to ambient light, quickly adapted by the mouse’s rods, making accurate measurement of the mutation’s effects challenging.
To overcome this, Johns Hopkins researchers genetically modified mice to exhibit a low expression of G90D, equivalent to 0.1% of normal rhodopsin levels found in natural mouse populations.
This adjustment allowed the researchers to differentiate between various activities produced in mice with the G90D mutation, simulating conditions with minimal or no background light.
Utilizing a high-resolution technique, they recorded the electrical activity in individual rods in the mouse retina, accessed via an ultra-fine glass pipette—about one-seventieth the width of a human hair—filled with saline solution to conduct electricity.
“You can actually see these events,” Yau remarked. “We employed a specialized method called suction-pipette recording to capture activity at such high resolution that even the isomerization of a single rhodopsin molecule is detectable due to the resulting change in electrical current.”
G90D is one of four rhodopsin mutations linked to night blindness. Chai, the first author, indicated that the next steps involve identifying how other rhodopsin mutations—T94I, A292E, and A295V—lead to this condition.
Chai suggested that the mechanism causing G90D night blindness might be analogous to those in the other three rhodopsin mutations.
Contributors to the research included Yaqing Ye, Daniel Silverman, and Randall Reed from Johns Hopkins, as well as Kasey Rose, Alana Madura, and Jeannie Chen from the University of Southern California. The study received funding from the National Institutes of Health grant EY006837, the António Champalimaud Vision Award (Portugal), the Multiphoton Imaging Core at Johns Hopkins, the Daniel Nathans Scientific Innovator Award from the Johns Hopkins University School of Medicine, and the Beckman-Argyros Vision Award from the Arnold and Mabel Beckman Foundation.
Follow us in Facebook
Discover more from An Eye Care Blog
Subscribe to get the latest posts sent to your email.

You must be logged in to post a comment.